Influence of Surfactant Hydrophobicity on Polycarbonate Membranes and its Effect on Permselectivity Stevie Bush Authors: Stevie Bush, Thomas Volta, Charles Martin |
|
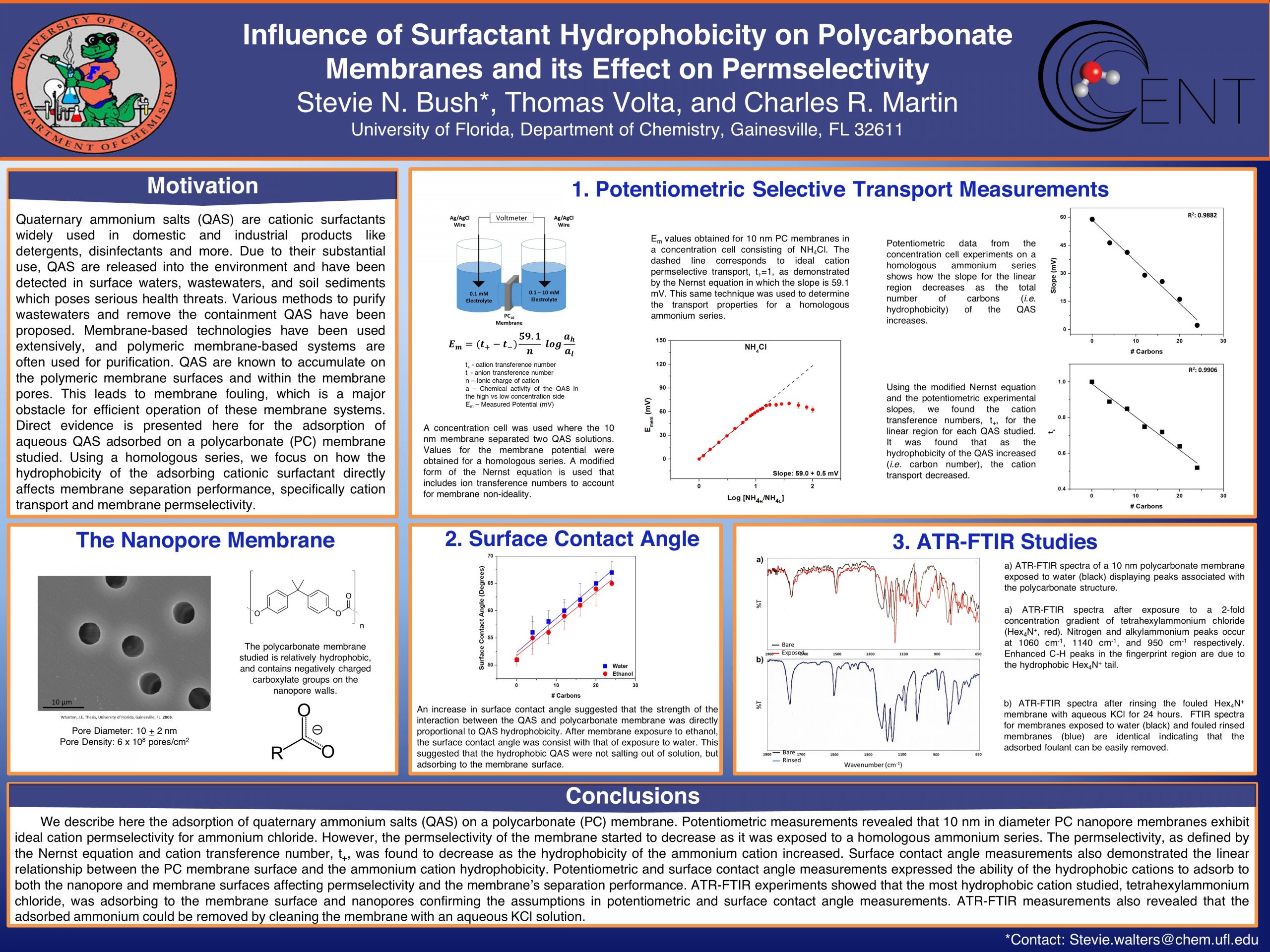
AbstractQuaternary ammonium salts (QAS) are cationic surfactants widely used in domestic and industrial products like detergents, disinfectants and more. Due to their substantial use, QAS are accidentally or intentionally released into the environment and have been detected in surface waters, wastewaters, and soil sediments, which pose serious health threats. Therefore, various methods to purify wastewaters and remove the containment QAS have been proposed. Membrane-based technologies have been used extensively, and wastewater treatment facilities often use polymeric membrane-based systems for purification. QAS are known to accumulate on the polymeric membrane surfaces and within the membrane pores. This leads to membrane fouling, which is a major obstacle for efficient operation of these membrane systems. Direct evidence is presented here for the adsorption of aqueous QAS adsorbed on a polycarbonate membrane studied. Potentiometric experiments, in accordance with surface contact angle and infrared spectroscopy measurements, illustrate the extent of adsorption on the membrane surface and within the membrane nanopores. Using a homologous quaternary ammonium series, we focus on how the hydrophobicity of the adsorbing cationic surfactant directly affects membrane separation performance. It was found that the strength of the interaction between the QAS and the polycarbonate membrane was directly proportional to QAS hydrophobicity. Excessive adsorption to the membrane surface and nanopore walls deteriorated membrane separation performance.
|
|
|
|
|
|
Poster Pitch
Click the video below to view the student’s poster pitch. |